
VORAPAXAR
Thrombosis, Antiplatelet Therapy, PAR1 Antagonists , MERCK ..ORIGINATOR
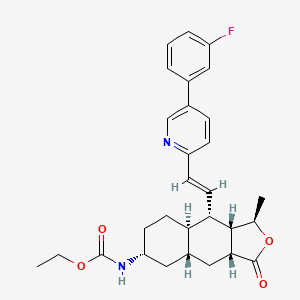
Ethyl N-[(3R,3aS,4S,4aR,7R,8aR,9aR)-4-[(E)-2-[5-(3-fluorophenyl)-2-pyridyl]vinyl]-3-methyl-1-oxo-3a,4,4a,5,6,7,8,8a,9,9a-decahydro-3H-benzo[f]isobenzofuran-7-yl]carbamate
618385-01-6 CAS NO
Also known as: SCH-530348, MK-5348
Molecular Formula: C29H33FN2O4
Molecular Weight: 492.581723
Vorapaxar (formerly SCH 530348) is a thrombin receptor (protease-activated receptor, PAR-1) antagonist based on the natural product himbacine. Discovered by Schering-Plough and currently being developed by Merck & Co., it is an experimental pharmaceutical treatment for acute coronary syndrome chest pain caused by coronary artery disease.[1]
In January 2011, clinical trials being conducted by Merck were halted for patients with stroke and mild heart conditions.[2] In a randomized double-blinded trial comparing vorapaxar with placebo in addition to standard therapy in 12,944 patients who had acute coronary syndromes, there was no significant reduction in a composite end point of death from cardiovascular causes, myocardial infarction, stroke, recurrent ischemia with rehospitalization, or urgent coronary revascularization. However, there was increased risk of major bleeding.[3]
A trial published in February 2012, found no change in all cause mortality while decreasing the risk of cardiac death and increasing the risk of major bleeding.[4]
SCH-530348 is a protease-activated thrombin receptor (PAR-1) antagonist developed by Schering-Plough and waiting for approval in U.S. for the oral secondary prevention of cardiovascular events in patients with a history of heart attack and no history of stroke or transient ischemic attack. The drug candidate is being investigated to determine its potential to provide clinical benefit without the liability of increased bleeding; a tendency associated with drugs that block thromboxane or ADP pathways. In April 2006, SCH-530348 was granted fast track designation in the U.S. for the secondary prevention of cardiovascular morbidity and mortality outcomes in at-risk patients.
Vorapaxar was recommended for FDA approval on January 15, 2014.[5]
 VORAPAXAR
VORAPAXAR
17 JAN 2014
FDA advisory panel votes to approve Merck & Co’s vorapaxar REF 6
FDA advisory panel votes to approve Merck & Co’s vorapaxar REF 6
VORAPAXAR SULPHATE

CAS Number: 705260-08-8
Molecular Formula: C29H33FN2O4.H2O4S
Molecular Weight: 590.7
Chemical Name: Ethyl [(1R,3aR,4aR,6R,8aR,9S,9aS)-9-[(1E)-2-[5-(3-fluorophenyl)pyridin-2- yl]ethenyl]-1-methyl-3-oxododecahydronaphtho[2,3-c]furan-6-yl]carbamate sulfate
Synonyms: Carbamic acid, [(1R,3aR,4aR,6R,8aR,9S,9aS)-9-[(1E)-2-[5-(3-fluorophenyl)-2- pyridinyl]ethenyl]dodecahydro-1-methyl-3-oxonaphtho[2,3-c]furan-6-yl]-,ethyl ester,sulfate; SCH-530348
Vorapaxar Sulfate (SCH 530348) a thrombin receptor (PAR-1) antagonist for the prevention and treatment of atherothrombosis.
........................
GENERAL INTRO
SIMILAR NATURAL PRODUCT
+ HIMBACINE

Himbacine is an alkaloid muscarinic receptor antagonist displaying more potent activity associated with M2 and M2 subtypes over M1 or M3. Observations show himbacine bound tightly to various chimeric receptors in COS-7 cells as well as possessed the ability to bind to cardiac muscarinic receptors allosterically. Recent studies have produced series of thrombin receptor (PAR1) antagonists derived from himbacine Himbacine is an inhibitor of mAChR M2 and mAChR M4.
Technical Information
| Physical State: | Solid |
| Derived from: | Australian pine Galbulimima baccata |
| Solubility: | Soluble in ethanol (50 mg/ml), methanol, and dichloromethane. Insoluble in water. |
| Storage: | Store at -20° C |
| Melting Point: | 132-134 °C |
| Boiling Point: | 469.65 °C at 760 mmHg |
| Density: | 1.08 g/cm3 |
| Refractive Index: | n20D 1.57 |
| Optical Activity: | α20/D +51.4º, c = 1.01 in chloroform |
| Application: | An alkaloid muscarinic receptor antagonist |
| CAS Number: | 6879-74-9 |
| Molecular Weight: | 345.5 |
| Molecular Formula: | C22H35NO2 |
general scheme:
.................................
SYNTHESIS
THE EXACT BELOW COMPD IS 14
Example 2
Step 1 :
Phosphonate 7, described in US 6,063,847, (3.27 g, 8.1 mmol) was dissolved in THF (12 ml) and C(O)Oled to 0 °C, followed by addition of 2.5 M n- BuLi (3.2 ml, 8.1 mmol). The reaction mixture was stirred at 0 °C for 10 min and warmed up to rt. A solution of aldehyde 6, described in US 6,063,847, in THF (12 ml) was added to the reaction mixture. The reaction mixture was stirred for 30 min. Standard aqueous work-up, followed by column chromatography (30-50% EtOAc in hexane) afforded product 8. 1HNMR (CDCI3): δ 0.92-1.38 (m, 31 H), 1.41 (d, J= 6 Hz, 3H), 1.40-1.55 (m, 2H), 1.70-1.80 (m, 2H), 1.81-1.90 (m, 2H), 2.36 (m, 2H), 2.69 (m, 1 H), 3.89 (m, 4H), 4.75 (m, 1 H), 6.28-6.41 (m, 2H), 7.05-7.15 (m, 2H), 8.19 (br s, 1 H). Step 2:
Compound 8 (2.64 g, 4.8 mmol) was dissolved in THF (48 ml). The reaction mixture was C(O)Oled to 0 °C followed by addition of 1 M TBAF (4.8 ml). The reaction mixture was stirred for 5 min followed by standard aqueous work-up. Column chromatography (50% EtOAc/hexane) afforded product 9 (1.9 g, 100%). 1HNMR (CDCI3): δ 1.15-1.55 (m, 6H), 1.41 (d, J= 6 Hz, 3H), 1.70-1.82 (m, 3H), 1.85-1.90 (m, 1 H), 2.36 (m, 2H), 2.69 (m, 1 H), 3.91 (m, 4H), 4.75 (m, 1 H), 6.18- 6.45 (m, 2H), 7.19 (br s, 2H), 8.19 (br s, 1 H). Step 3:
To a solution of compound 9 (250 mg, 0.65 mmol) in pyridine (5 ml) C(O)Oled to 0 °C was added Tf2O (295 μL, 2.1 mmol). The reaction mixture was stirred overnight at rt. Standard aqueous work-up followed by column chromatography afforded product 10 (270 mg, 80%). 1HNMR (CDCI3): δ 1.15-1.55 (m, 6H), 1.41 (d, J= 6 Hz, 3H), 1.70-1.82 (m, 3H), 1.85-1.90 (m, 1 H), 2.36 (m, 2H), 2.69 (m, 1 H), 3.91 (m, 4H), 4.75 (m, 1 H), 6.42-6.68 (m, 2H), 7.25 (m, 1 H), 7.55 (m, 1 H), 8.49 (d, J= 2.8 Hz, 1 H).
Compound 10 (560 mg, 1.1 mmol), 3-fluorophenyl boronic acid (180 mg, 1.3 mmol) and K2CO3 (500 mg, 3.6 mmol) were mixed with toluene (4.4 ml), H2O (1.5 ml) and EtOH (0.7 ml) in a sealed tube. Under an atmosphere of N2, Pd(Ph3P)4 (110 mg, 0.13 mmol) was added. The reaction mixture was heated at 100 °C for 2 h under N2. The reaction mixture was C(O)Oled down to rt, poured to EtOAc (30 ml) and washed with water (2X20 ml). The EtOAc solution was dried with NaHCO3 and concentrated at reduced pressure to give a residue. Preparative TLC separation of the residue (50% EtOAc in hexane) afforded product 11 (445 mg, 89%). 1HNMR (CDCI3): δ 1.15-1.59 (m, 6H), 1.43 (d, J= 6 Hz, 3H), 1.70-1.79 (m, 2H), 1.82 (m, 1H), 1.91 (m, 2H), 2.41 (m, 2H), 2.69 (m, 1 H), 3.91 (m, 4H), 4.75 (m, 1 H), 6.52-6.68 (m, 2H), 7.15 (m, 1 H), 7.22 (m, 2H), 7.35 (m, 1 H), 7.44 (m, 1 H), 7.81 (m, 1 H), 8.77 (d, J= 1.2 Hz, 1 H). Step 5:
Compound 11 (445 mg, 0.96 mmol) was dissolved in a mixture of acetone (10 ml) and 1 N HCI (10 ml). The reaction mixture was heated at 50 °C for 1 h.
Standard aqueous work-up followed by preparative TLC separation (50% EtOAc in hexane) afforded product 12 (356 mg, 89%). 1HNMR (CDCI3): δ 1.21-1.45 (m, 2H), 1.47 (d, J= 5.6 Hz, 3H), 1.58-1.65 (m, 2H), 2.15 (m, 1 H), 2.18-2.28 (m, 2H), 2.35- 2.51 (m, 5H), 2.71 (m, 1 H), 4.79 (m, 1 H), 6.52-6.68 (m, 2H), 7.15 (m, 1 H), 7.22 (m, 2H), 7.35 (m, 1 H), 7.44 (m, 1 H), 7.81 (m, 1 H), 8.77 (d, J= 1.2 Hz, 1 H). Step 6:
Compound 12 (500 mg, 4.2 mmol) was dissolved in EtOH (40 ml) and CH2CI2 (15 ml) NH3 (g) was bubbled into the solution for 5 min. The reaction mixture was C(O)Oled to 0 °C followed by addition of Ti(O/'Pr)4 (1.89 ml, 6.3 mmol). After stirring at 0 °C for 1 h, 1 M TiCI (6.3 ml, 6.3 mmol) was added. The reaction mixture was stirred at rt for 45 min and concentrated to dryness under reduced pressure. The residue was dissolved in CH3OH (10 ml) and NaBH3CN (510 mg, 8 mmol) was added. The reaction mixture was stirred overnight at rt. The reaction mixture was poured to 1 N NaOH (100 ml) and extracted with EtOAc (3x 100 ml). The organic layer was combined and dried with NaHC03. Removal of solvent and separation by PTLC (5% 2 M NH3 in CH3OH/ CH2CI2) afforded β-13 (spot 1 , 30 mg, 6%) and α-13 (spot 2, 98 mg, 20%). β-13: 1HNMR (CDCI3): δ 1.50-1.38 (m, 5H), 1.42 (d, J= 6 Hz, 3H), 1.51-1.75 (m, 5H), 1.84 (m, 2H), 2.38 (m, 1 H), 2.45 (m, 1 H), 3.38 (br s, 1 H), 4.78 (m, 1 H), 6.59 (m, 2H), 7.15 (m, 1 H), 7.26 (m, 2H), 7.36 (m, 1 H), 7.42 (m, 1 H), 7.82 (m, 1 H), 8.77 (d, J= 2 Hz, 1 H). α-13:1HNMR (CDCI3): δ 0.95 (m, 2H), 1.02-1.35 (m, 6H), 1.41 (d, J= 6 Hz, 3H), 1.82-1.95 (m, 4H), 2.37 (m; 2H), 2.69 (m, 2H), 4.71 (m, 1 H), 6.71 (m, 2H), 7.11 (m, 1 H), 7.25 (m, 2H), 7.38 (m, 1 H), 7.42 (m, 1 H), 7.80 (m, 1 H), 8.76 (d, J= 1.6 Hz, 1 H). Step 7:
Compound α-13 (300 mg, 0.71 mmol) was dissolved in CH2CI2 (10 ml) followed by addition of Et3N (0.9 ml). The reaction mixture was C(O)Oled to 0 °C and ethyl chloroformate (0.5 ml) was added. The reaction mixture was stirred at rt for 1 h. The reaction mixture was directly separated by preparative TLC (EtOAc/ hexane, 1 :1) to give the title compound (14) VORAPAXAR (300 mg, 86%). MS m/z 493 (M+1).
HRMS Calcd for C29H34N2O4F (M+1 ): 493.2503, found 493.2509.
.....................
SYNTHESIS 1
VORAPAXAR= COMPD A
Example 6 - Preparation of Compound A
To a three-neck flask equipped with an agitator, thermometer and nitrogen inertion was added 7A (13.0 g), THF (30 mL). The mixture was cooled to below -200C after which lithium diisopropylamide (2M, 20 mL) was slowly added. The reaction mixture was agitated for an additional hour (Solution A). To another flask was added 6 (10.0 g) and THF (75 mL) . The mixture was stirred for about 30 minutes and then slowly transferred into the solution A while maintaining the temperature below 200C. The mixture was stirred at below -200C for an additional hour before quenching the reaction by adding 20 mL of water. The reaction mixture was warmed to 00C and the pH was adjusted to about 7 by addition of 25% HaSO4 (11 mL). The mixture was further warmed to 200C and then diluted with 100 mL of ethyl acetate and 70 mL of water. The two phases that had formed were separated and the aqueous layer was extracted with 50 mL of ethyl acetate. The solvents THF and ethyl acetate were then replaced with ethanol, and the Compound A was precipitated out as a crystalline solid from ethanol with seeding at 35 to 4O0C. After cooling to O0C, the suspension was stirred for an additional hour and then the product was filtered and washed with cold ethanol. The product was dried at 50 - 600C under vacuum to provide an off-white solid. VORAPAXAR
Yield: 12.7 g, (90%). m.p. 104.90C (DSC onset point).
1H NMR (CDCl3) δ 8.88 (d, J = 2.4 Hz, IH), 8.10 (dd, J = 8.2, 2.4 Hz, IH), 7.64 (IH), 7.61 (d, J = 8.8 Hz, IH), 7.55 (m, J = 8.2, 6.2 Hz, IH), 7.51 (d, J = 8.0 Hz, IH), 7.25 (dt, J = 9.0, 2.3 Hz, IH), 7.08 (d, J = 8.0 Hz, IH), 6.68 (dd, J = 15.4, 9.4 Hz, IH), 6.58 (d, J = 9.6 Hz, IH), 4.85 (dd, J = 14.2, 7.2 Hz, IH), 3.95 (dd, J = 14.2, 7.1 Hz, 2H), 3.29 (m, IH), 2.66 (m, J = 12.0, 6.4 Hz, IH), 2.33 (m, 2H), 1.76 (m, 4H), 1.30 (d, J = 5.6 Hz, 3H), 1.19 (m, 4H), 1.14 (t, J = 7.2 Hz, 3H), 0.98 (m, IH), 0.84 (m, IH). MS (EI) m/z: calcd. 492, found 492.
BISULPHATE SALT
Example 7 - Preparation of an Acid Salt (bisulfate) of Compound A:
Compound IA (5 g) was dissolved in about 25 mL of acetonitrile.
The solution was agitated for about 10 minutes and then heated to about 50 0C. About 6 mL of 2M sulfuric acid in acetonitrile was added into the heated reaction mixture. The solid salt of Compound A precipitated out during the addition of sulfuric acid in acetonitrile. After addition of sulfuric acid solution, the reaction mixture was agitated for 1 hour before cooling to room temperature. The precipitated solid was filtered and washed with about 30 mL of acetonitrile. The wet solid was dried under vacuum at room temperature for 1 hour and at 80 0C for about 12 hours to provide about 5 g white solid (yield 85%). m.p. 217.0 0C. 1H NMR (DMSO) 9.04 (s, IH), 8.60 (d, J = 8.1 Hz, IH), 8.10 (d, J = 8.2 Hz, IH), 7.76 (d, J = 10.4, IH), 7.71 (d, J = 7.8 Hz, IH), 7.60 (dd, J = 8.4, 1.8 Hz, IH), 7.34 (dd, 8.4, 1.8 Hz, IH), 7.08 (d, J = 8.0 Hz, IH), 7.02 (m, IH), 6.69 (d, J = 15.8 Hz, IH), 4.82 (m, IH), 3.94 (dd, J = 14.0, 7.0 Hz, 2H), 3.35 (brs, IH), 2.68 (m, IH), 2.38 (m, 2H), 1.80-1.70 (m, 4H), 1.27 (d, J = 5.8 Hz, 3H), 1.21 (m, 2H), 1.13 (t, J = 7.0 Hz, 3H), 0.95 (m, IH, 0.85 (m, IH). MS (EI) m/z calcd. 590, found 492.
INTERMEDIATE 6
Example 5- Preparation of Compound 6
To a three-neck flask equipped with an agitator, thermometer and nitrogen inert were added the crude product solution of Compound 5 (containing about 31 g. of Compound 5 in 300 mL solution) and anhydrous DMF (0.05 mL). After the mixture was agitated for 5 minutes, oxalyl chloride (12.2 mL) was added slowly while maintaining the batch temperature between 15 and 25°C. The reaction mixture was agitated for about an hour after the addition and checked by NMR for completion of reaction. After the reaction was judged complete, the mixture was concentrated under vacuum to 135 mL while maintaining the temperature of the reaction mixture below 300C. The excess oxalyl chloride was removed completely by two cycles of vacuum concentration at below 500C with replenishment of toluene (315 mL) each time, resulting in a final volume of 68 mL. The reaction mixture was then cooled to 15 to 25°C, after which THF (160 mL) and 2,6-lutidine (22 mL) were added. The mixture was agitated for 16 hours at 20 to 25°C under 100 psi hydrogen in the presence of dry 5% Pd/C (9.0 g). After the reaction was judged complete, the reaction mixture was filtered through celite to remove catalyst. More THF was added to rinse the hydrogenator and catalyst, and the reaction mixture was again filtered through celite. Combined filtrates were concentrated under vacuum at below 25°C to 315 mL. MTBE (158 mL) and 10% aqueous solution of phosphoric acid (158 mL) were added for a thorough extraction at 100C to remove 2,6- lutidine. Then phosphoric acid was removed by extracting the organic layer with very dilute aqueous sodium bicarbonate solution (about 2%), which was followed by a washing with dilute brine. The organic solution was concentrated atmospherically to a volume of 90 mL for solvent replacement. IPA (315 mL) was added to the concentrated crude product solution. The remaining residual solvent was purged to <_ 0.5% of THF (by GC) by repeated concentration under vacuum to 68 mL, with replenishment of IPA (315 mL) before each concentration. The concentrated (68 mL) IPA solution was heated to 50°C, to initiate crystallization. To this mixture n-heptane (68 mL) was added very slowly while maintaining the batch temperature at 50°C. The crystallizing mixture was cooled very slowly over 2.5 hours to 25°C. Additional n- heptane (34 mL) was added very slowly into the suspension mixture at 250C. The mixture was further cooled to 200C, and aged at that temperature for about 20 hours. The solid was filtered and washed with a solvent mixture of 25% IPA in n-heptane, and then dried to provide
19.5 g of a beige colored solid of Compound 6. (Yield: 66%) m.p. 169.30C. IH NMR (CD3CN) δ 9.74 (d, J = 3.03 Hz, IH), 5.42 (br, IH), 4.69 (m, IH), 4.03 (q, J = 7.02 Hz, 2H), 3.43 (qt, J = 3.80, 7.84 Hz, IH), 2.67 (m, 2H), 2.50 (dt, J = 3.00, 8.52 Hz, IH), 1.93 (d, J = 12.0 Hz, 2H), 1.82 (dt, J = 3.28, 9.75 Hz, 2H), 1.54 (qd, J = 3.00, 10.5 Hz, IH), 1.27 (d, J = 5.97 Hz, 3H), 1.20 (m, 6H), 1.03 - 0.92 (m, 2H). MS (ESI) m/z (M++1): calcd. 324, found 324.
INTERMEDIATE 7A
Example 4 - Preparation of Compound 7A
+ 1-Pr2NLi + (EtO)2POCI - + LiCI
8
7A
To a 10 L three-necked round bottomed flask equipped with an agitator, thermometer and a nitrogen inlet tube, was added 20Og of
Compound 8 (1.07 mol, from Synergetica, Philadelphia, Pennsylvania). THF (1000 mL) was added to dissolve Compound 8. After the solution was cooled to -80 0C to -50 0C, 2.0 M LDA in hexane/THF(1175 mL, 2.2 eq) was added while maintaining the batch temperature below -50 0C. After about 15 minutes of agitation at -800C to -50 0C, diethyl chlorophosphate (185 mL, 1.2 eq) was added while maintaining the batch temperature below -50 0C. The mixture was agitated at a temperature from -800C to - 50 0C for about 15 minutes and diluted with n-heptane (1000 mL). This mixture was warmed up to about -35 0C and quenched with aqueous ammonium chloride (400 g in 1400 mL water) at a temperature below -10 0C. This mixture was agitated at -150C to -10 0C for about 15 minutes followed by agitation at 150C to 25 0C for about 15 minutes. The aqueous layer was split and extracted with toluene (400 mL). The combined organic layers were extracted with 2N hydrochloric acid (700 mL) twice. The product-containing hydrochloric acid layers were combined and added slowly to a mixture of toluene (1200 mL) and aqueous potassium carbonate (300 g in 800 mL water) at a temperature below 30 0C. The aqueous layer was extracted with toluene (1200 mL). The organic layers were combined and concentrated under vacuum to about 600 ml and filtered to remove inorganic salts. To the filtrate was added n-heptane (1000 ml) at about 55 0C. The mixture was cooled slowly to 40 0C, seeded, and cooled further slowly to -10 0C. The resulting slurry was aged at about -10 0C for 1 h, filtered, washed with n- heptane, and dried under vacuum to give a light brown solid (294 g, 85% yield), m.p. 52 0C (DSC onset point).1H NMR (CDCl3) δ 8.73 (d, J = 1.5 Hz, IH), 7.85 (dd, Ji = 8.0 Hz, J2 = 1.5 Hz, IH), 7.49 (dd, Ji = 8.0 Hz, J2 = 1.3 Hz, IH), 7.42 (m, IH), 7.32 (d, J = 7.8 Hz, IH), 7.24 (m, IH), 7.08 (dt, Ji = 8.3 Hz, J2 = 2.3 Hz, IH), 4.09 (m, 4H), 3.48 (d, J = 22.0 Hz, 2H), 1.27 (t, J = 7.0 Hz, 6H). MS (ESI) for M+H calcd. 324, found 324.
Example 3 - Preparation of Compound 5:
4 5
To a three-necked round bottomed flask equipped with an agitator, thermometer and a nitrogen inlet tube was added a solution of Compound 4 in aqueous ethanol (100 g active in 2870 ml). The solution was concentrated to about 700 ml under reduced pressure at 350C to 40°C to remove ethyl alcohol. The resultant homogeneous mixture was cooled to 200C to 300C and its pH was adjusted to range from 12 to 13 with 250 ml of 25% sodium hydroxide solution while maintaining the temperature at 20-300C. Then 82 ml of ethyl chloroformate was slowly added to the batch over a period of 1 hour while maintaining the batch temperature from 200C to 300C and aged for an additional 30 minutes. After the reaction was judged complete, the batch was acidified to pH 7 to 8 with 10 ml of concentrated hydrochloric acid (37%) and 750 ml of ethyl acetate. The pH of the reaction mixture was further adjusted to pH 2 to 3 with 35% aqueous hydrochloric acid solution. The organic layer was separated and the aqueous layer was extracted again with 750 ml of ethyl acetate. The combined organic layers were washed twice with water (200 ml) . Compound 5 was isolated from the organic layer by crystallization from ethyl acetate and heptane mixture (1: 1 mixture, 1500 ml) at about 700C to 80 0C. The solid was filtered at 500C to 60 °C, washed with heptane and then dried to provide an off-white solid (yield 50%). m.p. 197.7°C. 1HNMR (CD3CN) δ 5.31 (brs, IH), 4.67 (dt, J = 16.1, 5.9 Hz, IH), 4.03 (q, J = 7.1 Hz, 2H), 3.41 (m, IH), 2.55 - 2.70 (m, 2H), 1.87 - 1.92 (m, IH), 1.32 - 1.42 (m, IH), 1.30 (d, J = 5.92 Hz, 3H), 1.30 - 1.25 (m, 6H), 0.98 (qt, J = 15.7, 3.18 Hz, 2H). MS (ESI) M+l m/z calculated 340, found 340.
Example 2 - Preparation of Compound 4;
3 4
7.4 kg of ammonium formate was dissolved in 9L of water at 15- 250C, and then cooled to 0-100C. 8.9 kg of Compound 3 was charged at 0-150C followed by an addition of 89L of 2B ethyl alcohol. The batch was cooled to 0-50C 0.9 kg of 10% Palladium on carbon (50% wet) and 9 L of water were charged. The batch was then warmed to 18-280C and agitated for 5 hours, while maintaining the temperature between 18-28 0C. After the reaction was judged complete, 7 IL of water was charged. The batch was filtered and the wet catalyst cake was then washed with 8OL of water. The pH of the filtrate was adjusted to 1-2 with 4N aqueous hydrochloric acid solution. The solution was used in the next process step without further isolation. The yield is typically quantiative. m.p. 216.40C. IH NMR (D2O+1 drop HCl) δ 3.15 (m, IH), 2.76 (m, IH), 2.62 (m, IH), 2.48 (dd,J-5.75Hz, IH), 1.94 (m, 2H), 1.78 (m, 2H), 1.38 (m, 2H), 1.20 (m, 6H), 1.18 (m, IH), 0.98 (q,J=2.99Hz, IH).
Example 1 - Preparation of Compound 3
2B 3
To a reactor equipped with an agitator, thermometer and nitrogen, were added about 10.5 kg of 2B, 68 L of acetone and 68 L of IN aqueous hydrochloric acid solution. The mixture was heated to a temperature between 50 and 600C and agitated for about 1 hour before cooling to room temperature. After the reaction was judged complete, the solution was concentrated under reduced pressure to about 42 L and then cooled to a temperature between 0 and 50C. The cooled mixture was agitated for an additional hour. The product 3 was filtered, washed with cooled water and dried to provide an off-white solid (6.9 kg, yield 76%). m.p. 2510C. Η NMR (DMSO) δ 12.8 (s, IH), 4.72 (m, J = 5.90 Hz, IH), 2.58 (m, 2H), 2.40 (m, J = 6.03 Hz, 2H), 2.21 (dd, J = 19.0, 12.8 Hz, 3H), 2.05 (m, IH), 1.87 (q, J = 8.92 Hz, IH), 1.75 (m, IH), 1.55 (m, IH), 1.35 (q, J = 12.6 Hz, IH), 1.27 (d, J = 5.88 Hz, 3H). MS (ESI) M+l m/z calcd. 267, found 267.
NOTE
Compound 7A may be prepared from Compound 8 by treating Compound 8 with diethylchlorophosphate:
Compound 8 may be obtained by the process described by Kyoku, Kagehira et al in "Preparation of (haloaryl)pyridines," (API Corporation, Japan). Jpn. Kokai Tokkyo Koho (2004). 13pp. CODEN: JKXXAF JP
2004182713 A2 20040702. Compound 8 is subsequently reacted with a phosphate ester, such as a dialkyl halophosphate, to yield Compound 7A. Diethylchlorophosphate is preferred. The reaction is preferably conducted in the presence of a base, such as a dialkylithium amide, for example diisopropyl lithium amide.
.........................................
J Med Chem 2008, 51(11): 3061
The discovery of an exceptionally potent series of thrombin receptor (PAR-1) antagonists based on the natural product himbacine is described. Optimization of this series has led to the discovery of 4 (SCH 530348), a potent, oral antiplatelet agent that is currently undergoing Phase-III clinical trials for acute coronary syndrome (unstable angina/non-ST segment elevation myocardial infarction) and secondary prevention of cardiovascular events in high-risk patients.
Ethyl [(3aR,4aR,8aR,9aS)-9(S)-[(E)-2-[5-(3-fluorophenyl)-2-
pyridinyl]ethenyl]dodecahydro-1(R)-methyl-3-oxonaphtho[2,3-c]furan-6(R)-yl]carbamate (4).
pyridinyl]ethenyl]dodecahydro-1(R)-methyl-3-oxonaphtho[2,3-c]furan-6(R)-yl]carbamate (4).
4 (300 mg, 86%). MS m/z 493 (M+1).
HRMS Calcd for C29H34N2O4F
(M+1): 493.2503, found 493.2509; mp125 °C;
(M+1): 493.2503, found 493.2509; mp125 °C;
[ ]D20 6.6 (c 0.5, MeOH).
1HNMR (CDCl3):
0.88-1.18 (m, 5 H), 1.22-1.30 (m, 3 H), 1.43 (d, J = 5.85 Hz, 3 H), 1.88-2.10 (m, 4 H), 2.33-2.42 (m, 2 H),
2.75-2.67 (m, 1 H), 3.52-3.60 (m, 1 H), 4.06-4.14 (m, 2 H), 4.54-4.80 (m, 1 H), 4.71-4.77 (m, 1 H),
6.55-6.63 (m, 2 H), 7.07-7.12 (m, 1 H), 7.26-7.29 (m, 2 H), 7.34 (d, J = 8.05 Hz, 1 H), 7.41-7.46 (m, 1 H), 7.80-7.82 (m, 1 H), 8.76-8.71 (m, 1 H).
2.75-2.67 (m, 1 H), 3.52-3.60 (m, 1 H), 4.06-4.14 (m, 2 H), 4.54-4.80 (m, 1 H), 4.71-4.77 (m, 1 H),
6.55-6.63 (m, 2 H), 7.07-7.12 (m, 1 H), 7.26-7.29 (m, 2 H), 7.34 (d, J = 8.05 Hz, 1 H), 7.41-7.46 (m, 1 H), 7.80-7.82 (m, 1 H), 8.76-8.71 (m, 1 H).
..........................
References
- Samuel Chackalamannil; Wang, Yuguang; Greenlee, William J.; Hu, Zhiyong; Xia, Yan; Ahn, Ho-Sam; Boykow, George; Hsieh, Yunsheng et al. (2008). "Discovery of a Novel, Orally Active Himbacine-Based Thrombin Receptor Antagonist (SCH 530348) with Potent Antiplatelet Activity". Journal of Medicinal Chemistry 51 (11): 3061–4.doi:10.1021/jm800180e. PMID 18447380.
- Merck Blood Thinner Studies Halted in Select Patients, Bloomberg News, January 13, 2011
- Tricoci et al. (2012). "Thrombin-Receptor Antagonist Vorapaxar in Acute Coronary Syndromes". New England Journal of Medicine 366 (1): 20–33.doi:10.1056/NEJMoa1109719. PMID 22077816.
- Morrow, DA; Braunwald, E; Bonaca, MP; Ameriso, SF; Dalby, AJ; Fish, MP; Fox, KA; Lipka, LJ; Liu, X; Nicolau, JC; Ophuis, AJ; Paolasso, E; Scirica, BM; Spinar, J; Theroux, P; Wiviott, SD; Strony, J; Murphy, SA; TRA 2P–TIMI 50 Steering Committee and, Investigators (Apr 12, 2012). "Vorapaxar in the secondary prevention of atherothrombotic events.". The New England Journal of Medicine 366 (15): 1404–13. doi:10.1056/NEJMoa1200933.PMID 22443427.
- "Merck Statement on FDA Advisory Committee for Vorapaxar, Merck’s Investigational Antiplatelet Medicine". Merck. Retrieved 16 January 2014.
- http://www.forbes.com/sites/larryhusten/2014/01/15/fda-advisory-panel-votes-in-favor-of-approval-for-mercks-vorapaxar/
- SCH-530348 (Vorapaxar) is an investigational candidate for the prevention of arterial thrombosis in patients with acute coronary syndrome and peripheral arterial disease. "Convergent Synthesis of Both Enantiomers of 4-Hydroxypent-2-ynoic Acid Diphenylamide for a Thrombin Receptor Antagonist Sch530348 and Himbacine Analogues." Alex Zaks et al.: Adv. Synth. Catal. 2009, 351: 2351-2357 Full text;
- Discovery of a novel, orally active himbacine-based thrombin receptor antagonist (SCH 530348) with potent antiplatelet activity
J Med Chem 2008, 51(11): 3061
- Stu Borman (2005). "Hopes Ride on Drug Candidates: Researchers reveal potential new medicines for thrombosis, anxiety, diabetes, and cancer". Chemical & Engineering News 83 (16): 40–44.
PATENTS
- WO 2003089428
- WO 2006076452
- US 6063847
- WO 2006076565
- WO 2008005344
- WO2010/141525
- WO2008/5353
- US2008/26050
- WO2006/76564 mp, nmr
| US8138180 | 3-21-2012 | EXO-SELECTIVE SYNTHESIS OF HIMBACINE ANALOGS |
| US2011251392 | 10-14-2011 | EXO- AND DIASTEREO- SELECTIVE SYNTHESIS OF HIMBACINE ANALOGS |
| US7989653 | 8-3-2011 | Exo- and diastereo-selective syntheses of himbacine analogs |
| US2011065676 | 3-18-2011 | COMBINATION THERAPIES COMPRISING PAR1 ANTAGONISTS WITH NAR AGONISTS |
| US7772276 | 8-11-2010 | Exo-selective synthesis of himbacine analogs |
| US2010137597 | 6-4-2010 | SYNTHESIS Of DIETHYLPHOSPHONATE |
| US7713999 | 5-12-2010 | THROMBIN RECEPTOR ANTAGONISTS |
| US7687631 | 3-31-2010 | Synthesis of diethyl{[5-(3-fluorophenyl)-pyridine-2yl]methyl}phosphonate |
| US2009297576 | 12-4-2009 | Local Delivery of PAR-1 Antagonists to Treat Vascular Complications |
| US7626045 | 12-2-2009 | SYNTHESIS OF HIMBACINE ANALOGS |
| US7605275 | 10-21-2009 | Exo- and diastereo- selective syntheses of himbacine analogs |
| US7553987 | 6-31-2009 | Synthesis of 3-(5-nitrocyclohex-1-enyl) acrylic acid and esters thereof |
| US7541471 | 6-3-2009 | Synthesis of himbacine analogs |
| US2009022729 | 1-23-2009 | METHODS AND COMPOSITIONS FOR TREATING CARDIAC DYSFUNCTIONS |
| US2008234236 | 9-26-2008 | REDUCTION OF ADVERSE EVENTS AFTER PERCUTANEOUS INTERVENTION BY USE OF A THROMBIN RECEPTOR ANTAGONIST |
| US2008031943 | 2-8-2008 | IMMEDIATE-RELEASE TABLET FORMULATIONS OF A THROMBIN RECEPTOR ANTAGONIST |
| US2008026050 | 1-32-2008 | SOLID DOSE FORMULATIONS OF A THROMBIN RECEPTOR ANTAGONIST |
| US7304078 | 12-5-2007 | Thrombin receptor antagonists |
| US2007270439 | 11-23-2007 | THROMBIN RECEPTOR ANTAGONISTS |
| US2007202140 | 8-31-2007 | THROMBIN RECEPTOR ANTAGONISTS AS PROPHYLAXIS TO COMPLICATIONS FROM CARDIOPULMONARY SURGERY |
| US2007203193 | 8-31-2007 | CRYSTALLINE POLYMORPH OF A BISULFATE SALT OF A THROMBIN RECEPTOR ANTAGONIST |
| US7235567 | 6-27-2007 | Crystalline polymorph of a bisulfate salt of a thrombin receptor antagonist |
| US2006172397 | 8-4-2006 | Preparation of chiral propargylic alcohol and ester intermediates of himbacine analogs |
| US2004192753 | 9-31-2004 | Methods of use of thrombin receptor antagonists |
| US6063847 * | Nov 23, 1998 | May 16, 2000 | Schering Corporation | Thrombin receptor antagonists |
| US6326380 * | Apr 7, 2000 | Dec 4, 2001 | Schering Corporation | Thrombin receptor antagonists |
| US20030216437 * | Apr 14, 2003 | Nov 20, 2003 | Schering Corporation | Thrombin receptor antagonists |
| US20040176418 * | Jan 9, 2004 | Sep 9, 2004 | Schering Corporation | Crystalline polymorph of a bisulfate salt of a thrombin receptor antagonist |
| WO2011128420A1 | Apr 14, 2011 | Oct 20, 2011 | Sanofi | Pyridyl-vinyl pyrazoloquinolines as par1 inhibitors |

















No comments:
Post a Comment