Atomoxetine

Atomoxetine
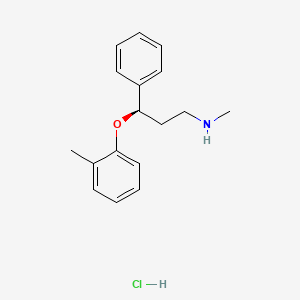
Atomoxetine hydrochloride (CAS NO.: 82248-59-7)
(R)-(-)-N-Methyl-gamma-(2-methylphenoxy)benzenepropanamine hydrochloride
| PATENT NO | PATENTEXPIRY DATE | |
|---|---|---|
| 5658590 | Nov 26, 2016 | |
| 5658590*PED | May 26, 2017 |
nda 021411 app 2002-11-26
TREATMENT OF ATTENTION-DEFICIT HYPERACTIVITY DISORDER
label
| COUNTRY | PATENT NUMBER | APPROVED | EXPIRES (ESTIMATED) |
|---|---|---|---|
| United States | 5658590 | 1997-05-26 | 2017-05-26 |
The HCl salt of atomoxetine , with the (R)-configuration], which is marketed under the trade name Strattera, is used for treating attention-deficit hyperactivity disorder (ADHD
Atomoxetine is a drug approved for the treatment of attention-deficit hyperactivity disorder(ADHD).[1] It is a selective norepinephrine reuptake inhibitor (NRI),[1] not to be confused with serotonin norepinephrine reuptake inhibitors (SNRIs) or selective serotonin reuptake inhibitors (SSRIs), both of which are currently the most prescribed form of antidepressants.
his compound is manufactured, marketed and sold in theUnited States under the brand name Strattera by Eli Lilly and Company as a hydrochloride salt (atomoxetine HCl), the original patent filing company, and current U.S. patent owner. Generics of atomoxetine are sold in all other countries; they are manufactured by Torrent Pharmaceuticals using the label Tomoxetin, Ranbaxy Laboratories (through its Division: Solus) using the label Attentin, Sun Pharmaceuticals(through its Division: Milmet Pharmaceuticals), and Intas Biopharmaceuticals There is currently no generic manufactured directly in the United States since it is under patent until 2017.[2]
On August 12, 2010, Lilly lost a lawsuit that challenged Lilly’s patent on Strattera, increasing the likelihood of an earlier entry of a generic into the US market.[3] On September 1, 2010, Sun Pharmaceuticals announced it would begin manufacturing a generic in the United States.[4] In a July 29, 2011 conference call, however, Sun Pharmaceutical’s Chairman stated “Lilly won that litigation on appeal so I think [generic Strattera]’s deferred.”[5]
Atomoxetine is designated chemically as (−)-N-methyl-3-phenyl-3-(o-tolyloxy)-propylamine hydrochloride, and has a molecular mass of 291.82.[1] It has a solubility of 27.8 mg/mL in water.[1] Atomoxetine is a white solid that exists as a granular powder inside the capsule, along with pre-gelatinized starch and dimethicone.[1] The capsule shells contain gelatin, sodium lauryl sulfate, FD&C Blue No. 2, synthetic yellow iron oxide, titanium dioxide, red iron oxide, edible black ink, and trace amounts of other inactive ingredients.[1]
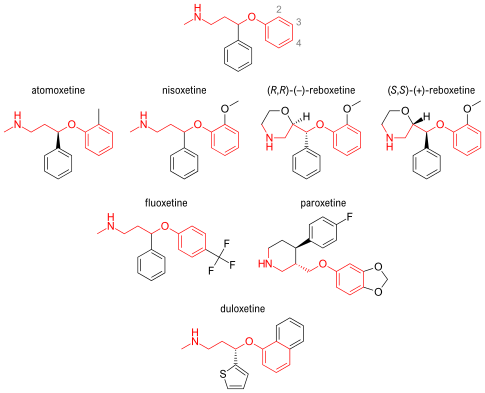
The compound (-)-N-methyl-3-(2-methylphenoxy)-3-phenylpropylamine, or (-)-Λ/-methyl-3-phenyl-3-(o-tolyloxy)-propylamine hydrochloride, is usually known by its adopted name “atomoxetine hydrochloride.” It is represented as shown in Formula 1 and is a selective norepinephrine reuptake inhibitor. A commercialatomoxetine hydrochloride product is sold as STRATTERA™ in the form of capsules containing 10, 18, 25, 40, 60, 80, or 100 mg of atomoxetine, for treating attention-deficit/hyperactivity disorder.
- “STRATTERA® (atomoxetine hydrochloride) CAPSULES for Oral Use. Full Prescribing Information.” Eli Lilly and Company, 2002, 2013. Revised August 5, 2013. [1]
- “Patent and Exclusivity Search Results”. Electronic Orange Book. US Food and Drug Administration. Retrieved 26 April 2009.
- “Drugmaker Eli Lilly loses patent case over ADHD drug, lowers revenue outlook”. Chicago Tribune.
- “Sun Pharma receives USFDA approval for generic Strattera capsules”. International Business Times.
- “Sun Pharma Q1 2011-12 Earnings Call Transcript 10.00 am, July 29, 2011″.
- Strattera by Eli Lilly and Company
- RxList.com – Strattera
- Detailed Strattera Consumer Information: Uses, Precautions, Side Effects
- All disclosed Lilly trials
- MSDS for Atomoxetine HCl
- Strattera Related Published Studies
Synthesis
Also known as: Atomoxetine hydrochloride, Strattera, Atomoxetine HCL, (R)-Tomoxetine hydrochloride, TOMOXETINE HYDROCHLORIDE, Tomoxetine, 82248-59-7
Atomoxetine is the first non-stimulant drug approved for the treatment of attention-deficit hyperactivity disorder (ADHD). It is sold in the form of the hydrochloride salt of atomoxetine. This chemical is manufactured and marketed under the brand name Strattera; by Eli Lilly and Company and as a generic Attentin by Torrent Pharmaceuticals. There is currently no generic available within the United States due to patent restrictions.
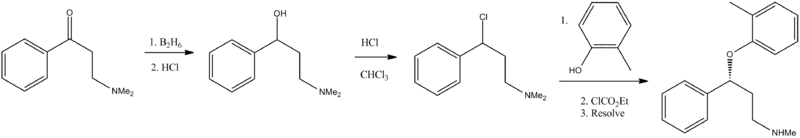
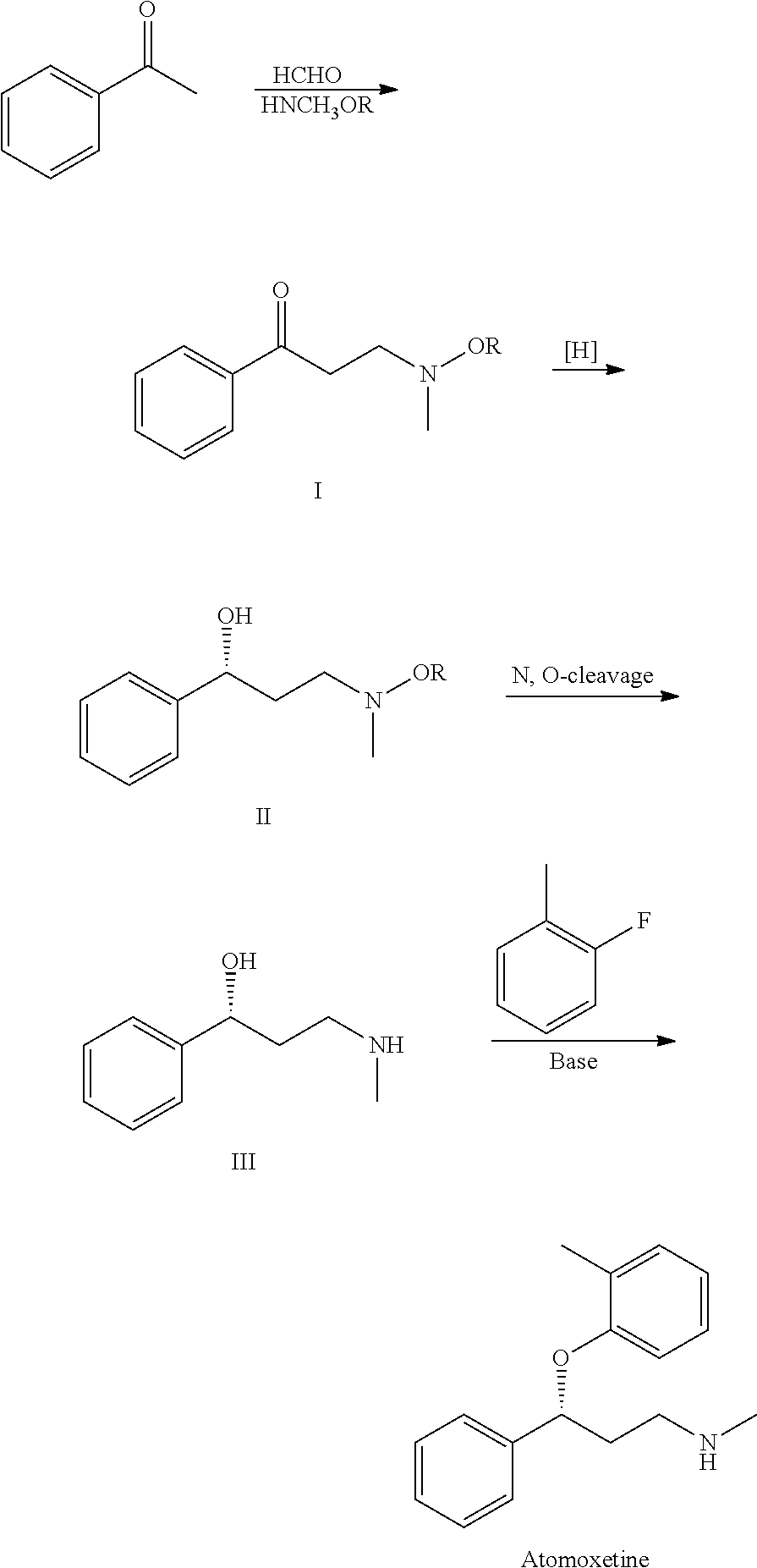
First step appears to be a Mannich reaction between acetophenone, paraformaldehyde and dimethylamine, although not formally written in the scheme.
Foster, B. J.; Lavagnino, E. R.; European Patent, 1982, EP 0052492.


Atomoxetine, designated chemically as (-)-N-methyl-3-phenyl-3-(0-tolyloxy)- propylamine hydrochloride, is structurally represented by the compound of Formula-I and is indicated for the potential treatment of attention-deficit hyperactivity disorder (ADHD). This compound is manufactured, marketed and sold in the United States under the brand name Strattera.
Formula-I Atomoxetine was first disclosed in US Patent No 4314081. The said patent disclosed Atomoxetine, its pharmaceutically acceptable salts and composition containing them.
4-hydroxy Atomoxetine, chemically known as R-(-)-N-methyl-3-(2-methyl-4- hydroxyphenyl)oxy)-3 -phenyl- 1-aminopropane, structurally represented by Formula-II, is a metabolite of Atomoxetine.
Fomnula-ll
4-hydroxy Atomoxetine hydrochloride was first disclosed in US Patent No 7384983, wherein 4-hydroxy Atomoxetine free base was dissolved in ethylacetate, treated the solution with 0.1N HC1; followed by lyophilization yielded a yellow solid which was dissolved in methanol and passed through a short column of activated carbon; the solvent was removed and finally the hydrochloride salt was recrystallized from water to afford 4-hydroxy Atomoxetine hydrochloride. However, this patent does not mention about the nature of the polymorph obtained through this process.
The asymmetric epoxidation of (E)-3-phenyl-2-propen-1-ol (I) by means of titanium tetraisopropoxide, (+)-diethyl tartrate (+)-(DET) and tBu-OOH in dichloromethane gives the chiral epoxide (II), which is opened by means of bis(2-methoxyethoxy)aluminum hydride (Red-Al) in DME to yield the chiral diol (III). The regioselective reaction of (III) with Ms-Cl and TEA in ethyl ether affords the primary mesylate (IV), which is condensed with 2-methylphenol (V) by means of PPh3 and DEAD in ethyl ether to provide the adduct (VI). Finally this compound is treated with methylamine in hot aq. THF to give rise to the target (R)-tomoxetine.

The reduction of omega-chloropropiophenone (I) with NaBH4 in ethanol gives 3-chloro-1-phenyl-1-propanol (II), which is treated with butyric anhydride and pyridine in dichloromethane to yield the corresponding racemic ester (III). The optical resolution of (III) with immobilized lipase B from Candida antarctica (CALB) affords a mixture of unreacted (S)-ester and (R)-alcohol (IV) that are separated by column chromatography. Condensation of th (R)-alcohol (IV) with 2-methylphenol (V) by means of PPh3 and diethyl azodicarboxylate (DEAD) in THF gives the corresponding ether (VI), which is finally treated with methylamine in refluxing ethanol.
more info
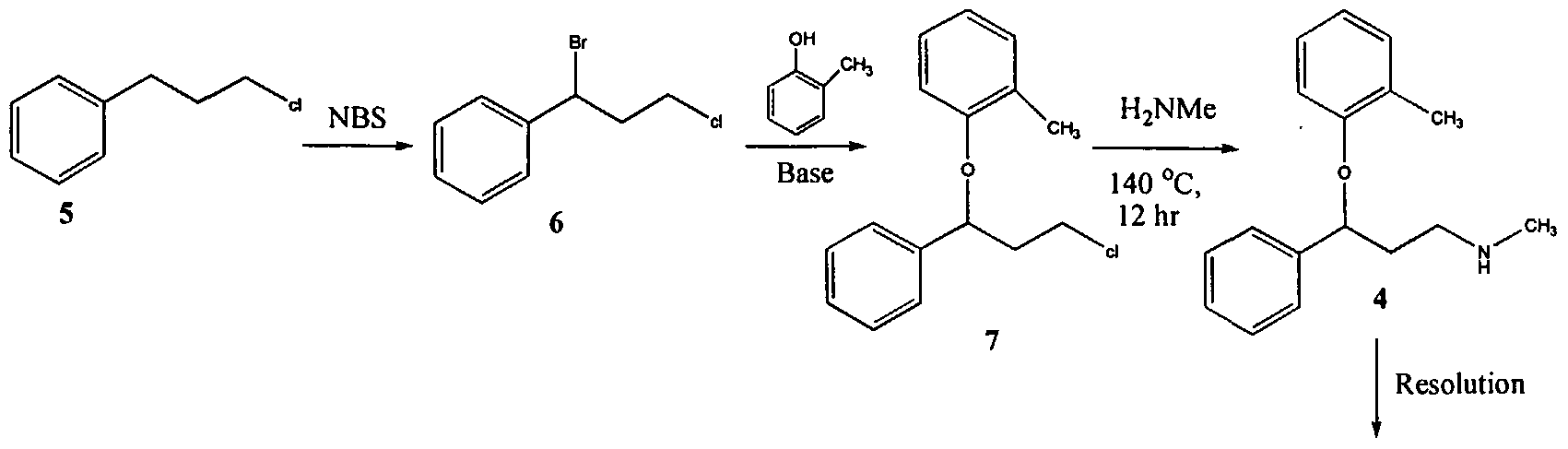
U.S. Patent No. 4,314,081 describes 3-Aryloxy-3-phenyl polyamines, which possess central nervous system activity. Atomoxetine is a member of the above class of compounds, and is a useful drug for the treatment of depression.Atomoxetine was claimed in U. S. Patent No. 4,314,081 and the patent describes a process for the preparation of atomoxetine and related compounds in two different ways as depicted below as Scheme A and Scheme B, respectively.
Scheme A
Atomoxetinc
Scheme B
The process illustrated in Scheme A involves the preparation of the atomoxetineusing 3-phenyl chloropropyl amine (Formula 5) as a starting material. The process involves bromination of said starting compound (Formula 5) by using N-bromosuccinimide. Further the bromo derivative is condensed with o-cresol to result in a compound of Formula 7, which is then subjected to amination using methylamine. Though the process looks very simple, it involves the following disadvantages: i) N-bromosuccinimide being a corrosive and sensitive chemical, its usage demands special care; ii) the workup of the compound formula 7 involves high vacuum (0.03 torr) distillation at 135-1450C, which is a tedious and cumbersome process to carry out at the plant level; and iii) the reaction conditions involved in some of the steps are harsh, for example the amination reaction is conducted at 14O0C. at pressures of 10 kg/cm2 for 12 hours in an autoclave.
All the above points make the process not viable for practicing on a commercial scale. Further, as described in U.S. Patent 4,314,081 , the free base compounds exist as high boiling oils, but form white crystalline salts.
On the other hand, Scheme B describes the preparation of atomoxetine using β-dimethylaminopropiophenone produced by a Mannich reaction; which is reduced to the hydroxy derivative having Formula 9 using diborane; further the hydroxy compound (Formula 9) is converted to the corresponding chloro derivative of Formula 10 using dry HCI gas and thionyl chloride and is followed by condensation with o-cresol.
The said reaction is carried out in methanol at reflux for a duration of five days to achieve the compound of formula 11 and is followed by demethylation using cyanogen bromide to end up with atomoxetine. As can be clearly understood the process is associated with the following problems: i) the use of costly reagents such as diborane makes the process uneconomical; ii) the passage of dry HCI gas followed by thionyl chloride addition is ^ very cumbersome and is not advisable in the plant; iii) this is a time-consuming process, involving a reaction which requires five days for its completion; and iv) use of cyanogen bromide, which is highly toxic, is not desirable.
All of the above-quoted drawbacks make the process unfriendly to practice in a production plant as well as to the environment.
Further, M. Srebnik et al., Journal of Organic Chemistry, Vol. 53, pages2916-2920 (1988); E. Corey et al., Tetrahedron Letters, Vol. 30, pages 5207-5210 (1989);
U.S. Patent No. 4,868,344; Y. Gao et al., Journal of Organic Chemistry, Vol. 53, pages 4081-4084 (1988); J. Deeter et al.,
Tetrahedron Letters, Vol. 31, pages 7101-7104 (1990);
and U.S. Patent No. 4,950,791 disclose stereospecific methods for the preparation of 3-aryloxy-3-phenylpropylamines; the enantiomers of 3-hydroxy-3-phenylpropylamines are prepared by the stereospecific reduction of the corresponding ketones. The thus obtained (S)-3-hydroxy-3-phenyl propylamines are subjected to condensation with aryl alcohols using the Mitsunobo reaction. As can be seen in Scheme C, the reaction involves two critical steps.
Scheme C
Dusopinocampheny) chloroborane
OH
,CH,
DEAD/ tn phenyl phosphine
The first critical step is an asymmetric reduction of the ketone to its corresponding alcohol. The second critical step involves the condensation of the obtained enantiomeric alcohol with the corresponding aryl alcohol. The process suffers from the following disadvantages:
1) the reagent used for the asymmetric reduction of the ketone is highly expensive;
2) the reagent diethyl azodicarboxylate (“DEAD”) is expensive;
3) the DEAD reagent is known to be highly carcinogenic, thus creating problems in handling; and
4) the reaction involves the use of triphenylphosphine and DEAD and the resulting byproducts formed in the reaction, phoshineoxide and a hydrazine derivative, are very difficult to remove.
Therefore, commercial applicability of the said process is limited owing to the above noted disadvantages.
International Patent Publication No. WO 00/58262 relates to a stereo- specific process for the preparation of atomoxetineusing nucleophilic aromatic displacement of an aromatic ring having a functional group, which can be converted to a methyl group. As can be seen, the process is very lengthy and involves many steps and is thus not commercially desirable.
U.S. Patent No. 5,847,214 describes the nucleophilic aromatic displacement reaction of 3-hydroxy-3-arylpropylamines with activated aryl halides, for example the reaction of N-methyl-3-phenyl-3-hydroxypropylamine with 4- triflouromethyl-1-cholro benzene has been reported; the success of this reaction is mainly due to electron withdrawing group on benzene ring of the aryl halides.
U.S. Patent No. 6,541 ,668 describes a process for the preparation of atomoxetine and its pharmaceutically acceptable addition salts which comprises reacting an alkoxide of N-methyl-3-phenyl-3-hydroxy propyl amine or an N protected derivative thereof, with 2-flouro toluene in the presence of 1 ,3-Dimethyl – 2-imidazolidinone (“DMI”) or N-Methyl-3-pyrrolidinone (“NMP”) as the solvent. The process disclosed in the said patent can be shown as Scheme D. Further, the process disclosed in the said patent restricts itself to the solvents DMI and NMP.
Scheme D
Nevertheless, a new crystalline form of N-methyl-3-phenyl-3-(o- tolyloxy)propylamine oxalate and an isolation technique of (±)-atmoxetine free base in a solid form, an intermediate useful in the synthesis of atomoxetine hydrochloride, is desirable.
There have been several methods reported for preparing (R)-(−)-N-methyl-3-(2-methylphenoxy)-3-phenylpropylamine (Atomoxetine®). For example, U.S. Pat. No. 4,868,344 discloses a process as shown in the following scheme:
In this example, 3-chloropropiophenone is used as the starting material to be asymmetrically reduced with (−)-diisopinocamphenylchloroborane ((−)-IPc2BC1) to give the corresponding chiral alcohol. The resulting chiral alcohol is then reacted with o-cresol via Mitsunobu reaction to form the chiral ether compound. Subsequently, amination of the chiral ether compound with methylamine provided atomoxetine. In this process, the materials such as chiral-borane ((−)-IPc2BC1) and diethyl azodicarboxylate (DEAD) are expensive, and result in high manufacturing cost.
Further, WO 2006/009884 discloses another method for preparing atomoxetine, including the step of reacting N-methyl-3-phenyl-3-hydroxypropylamine with 2-fluorotoluene which is followed by resolution of the resulting product to provide optically pure atomoxetine as shown in the following scheme:
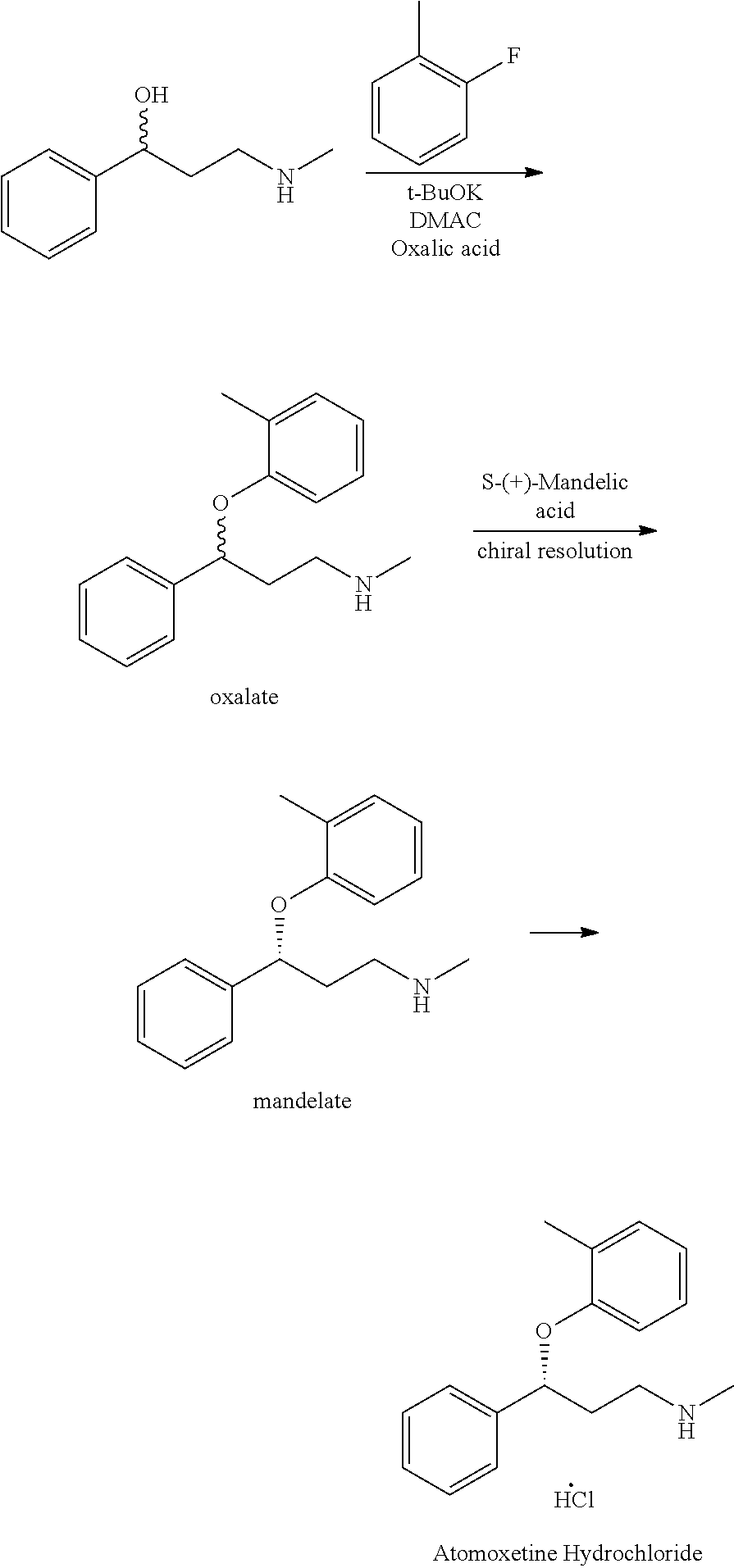
This process involving a chiral resolution step is inefficient due to low product yield, complicated and long time process that renders this process economically less competitive.
………………………………………………………………………………………………
see below
B.-F. Chen and co-inventors describe a synthesis of 5 that avoids costly reagents. It includes the preparation of the chiral amino alcohol 3 as a key intermediate. The route for preparing 5 starts with a Mannich reaction between benzophenone, N,O-dimethylhydroxylamine, and paraformaldehyde to give compound 1, isolated in 87.6% yield.
Ketone 1 is asymmetrically reduced to form alcohol 2 by using the chiral ruthenium catalyst RuCl2-[(S)-DMSEGPHOS)][(S)-DAIPEN]. The hydrogen pressure is described as “predetermined”, but no value is given. Product 2 is recovered as an oily product in 98.7% yield with 98.8% purity and 99% ee. It appears that the catalyst is not removed before the next step in which the oil is hydrogenated over a Raney nickel catalyst to form amino alcohol 3.
Intermediate 3 is also isolated as an oil in 96.4% yield, 96.5% % purity, and 99% ee. After recrystallization from toluene–heptane, the solid product is recovered with 100% ee. In the last step, 3 is treated with fluorotoluene 4 in the presence of t-BuOK to form atomoxetine, isolated as an oil in 91% yield with 97% ee. The purification of 5 and its conversion to the HCl salt are not described.
The inventors provide basic 1H-NMR data for all compounds except 5. The example describing the preparation of 1 lists one of the reactants as 2-acetylthiophene, which is clearly incorrect; and another reagent is called “32% hydrochloride”. These errors should have been spotted by anyone with a fundamental knowledge of chemistry who was involved in writing the patent—perhaps none were. (Sci Pharmtech [Taiwan]. US Patent 8,299,305, Oct. 30, 2012; Keith Turner)
View the full-text patent here.
| Patent Number: | US 8299305 |
| Title: | Process for preparation of atomoxetine |
| Inventor(s): | Chen, Bo-Fong; Li, Yan-Wei; Yeh, Jinun-Ban; Wong, Wei-Chyun |
| Patent Assignee(s): | SCI Pharmtech, Inc., Taiwan |









No comments:
Post a Comment